| Buffers |
A buffer is a substance that can give or accept protons i.e. hydrogen ions, in a manner that tends to minimise changes in the pH of the solution. Usually buffers are composed of a weak acid (proton donor) and a weak base (proton acceptor) as shown in the following equation.
![]()
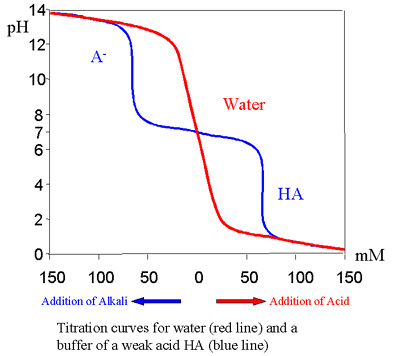
As shown in the titration curves, addition of strong ions at the alkaline end where only A- is present or at the acidic end, where only HA is present, the buffer solution acts like water - a small addition produces large changes in the pH - no buffering activity. However within about + 1 pH unit of the pKa (negative logarithm of the dissociation constant of the weak acid), an addition of a relatively large quantity of strong ions had very little effect on the pH of the solution.
Chemical buffers are the first line of defense of the body against drastic changes in the pH. Important buffers include:
| 1. | Carbonic acid/bicarbonate system - direct relation to amount of carbon dioxide dissolved in the blood |
| 2. | Phosphate system - especially important intracellularly |
| 3. | Proteins - important both intra and extracelllularly as being large molecules each with its own pKa, can buffer within a very large area of pH change. |