Acid Base Balance
The buffering capacity of the blood is composed of a number of systems that include
1.
Hemoglobin
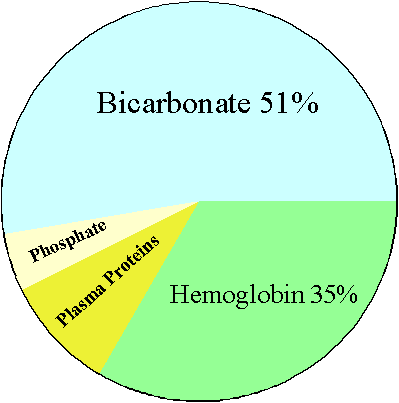
2.
Plasma proteins
3.
Phosphate system
4.
Bicarbonate system
The acidity of the blood is constantly being threatened both by the process of respiration as well as by the processes of metabolism. Hydrogen ions are primarily buffered by the buffer systems. In addition, the bicarbonate buffer system has an added advantage of the others, as it can be modified through the respiratory system. Thus during periods of increase acid output (acidosis), the respiratory system is stimulated so that more carbon dioxide diffuses out with a resultant decrease in acidity.
On the contrary, during episodes of increased alkalinity (alkalosis), the respiratory system is depressed so the PCO2 of the blood increases. This results in a decrease in alkalinity.
Though both these mechanisms would tend to bring back the pH to normal levels, in both cases the pH is only compensated as the changes occur at the cost of a change in bicarbonate pool. Final and definite adjustments of the pH occurs at the renal level as the kidney has the ability to excrete either an alkaline urine or an acidic urine and thus it can excrete or reabsorb hydrogen ions.