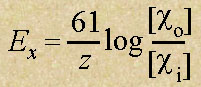- If the membrane was solely
permeable to potassium the magnitude of the
potential difference would be -90mV.
- The negative sign infront of the
potential difference, indicates that the
intracellular space is negative in respect to the
extracellular space.
- Another way of looking at this
difference is that an electrical potential that
is exactly equal but of opposite charge to the
membrane potential would block the diffusion of
the particular ion involved.
- Since the magnitude of the
diffusion depends on ratio of the concnetrations
between the extracellular and intracellular
space, then an equation can be built to calculate
this potential.
- This is called the Nernst
Equation.
- A simplified equation at room
temperature:
|
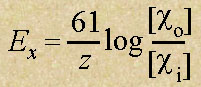
|
| where |
| |
Ex |
= |
equilibrium potential in millivolts
(mV) for ion x |
| |
co |
= |
concentration of the ion outside the
cell |
| |
ci |
= |
concentration of the ion outside the
cell |
| |
z |
= |
Valence of the ion (+1 for Na+ or K+) |
| |
|
|
|
- Using the Nernst Equation the equilibrium
potential for a cation is negative when the
internal concentration is higher than the
external concnetration. Thus if the membrane is
fully permeable to potassium, then the membrane
potential would be negative whilst if the
membrane was solely permeable to sodium, then the
potential would be positive.
- Thus in the case of the cellular resting membrane
potential, the Potassium Equilibirum Potential is
-90mV. If the membrane was fully permeable to
Sodium, then the Sodium Equilibrium Potential
would be +60mV.
|