|
The category of molecules under
the title of lipids, inlcude a diverse range of
molecules that differ greatly in chemical
composition but have one common characteristic
i.e. they are insoluble in water.
This lack of solubility is due to
the fact that lipids consist of hydrocarbon
chains and rings which are non-polar.
|
- Free Fatty Acids (FFA) consist of
a non-polar hydrocarbon chain and a carboxyl
(COOH) group.
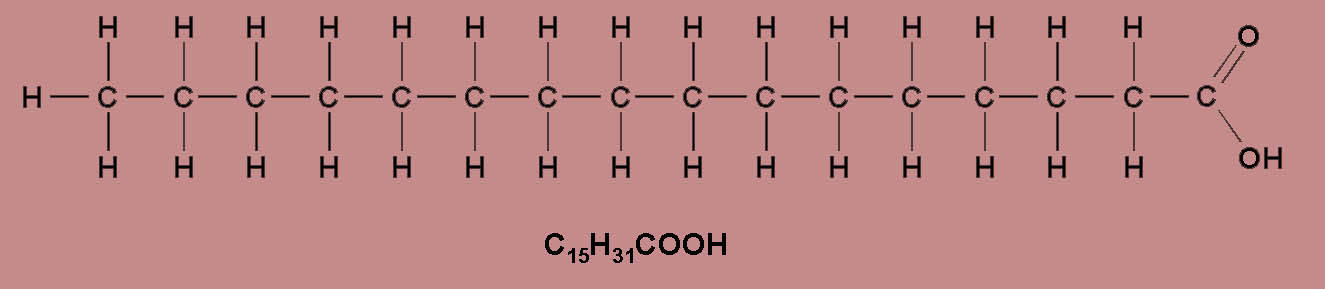
- If all the carbon atoms within the
hydrocarbon chain are joined together by a single
co-valent bond, than the FFA is said to be fully
saturated, e.g. Palmitic Acid, C15H31COOH.
|
 |
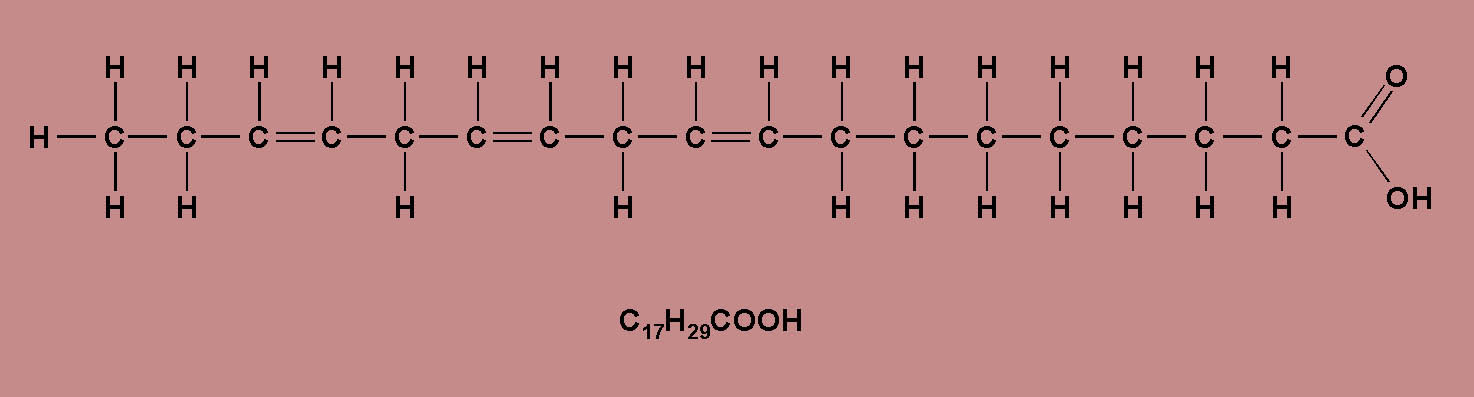
- If on the other hand, one or more
pairs of carbon atoms in the hydrocarbon chain
are joined by double co-valent bonds, than the
FFA is said to be unsaturated.
|
 |

